Louvain-la-Neuve, Belgium, 7 May 2022 - IBA (Ion Beam Applications S.A., EURONEXT), the world leader in particle accelerator technology and a world leading provider of dosimetry solutions, today announces that it will share the latest clinical research on myQA® SRS, its film-class resolution stereotactic detector array, and MatriXX ResolutionTM, the highest resolution IMRT/VMAT QA detector, at ESTRO 2022.
One year after the release of its high-resolution detectors myQA® SRS and MatriXX Resolution, IBA Dosimetry is sharing its extensive research related to innovation in patient QA, with more than 20 shared customer’s experiences and publications in the past year. Four publications will be presented at ESTRO and can be found in the conference program and Abstract book.
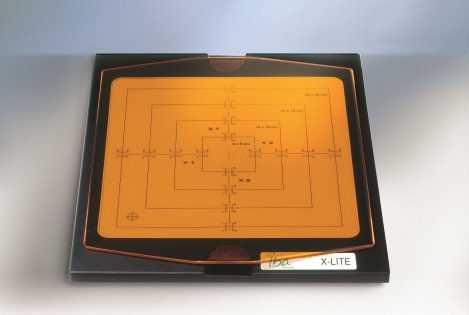
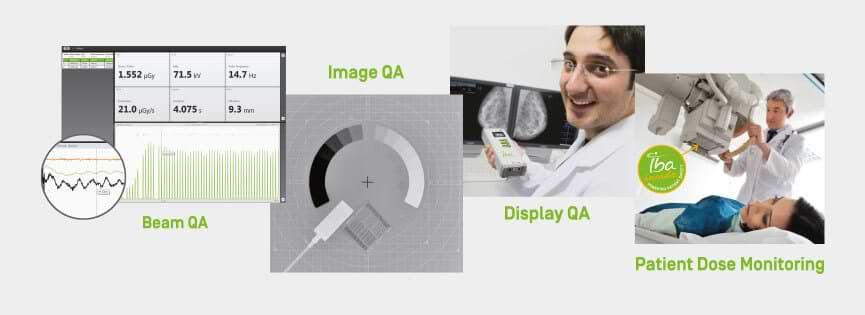
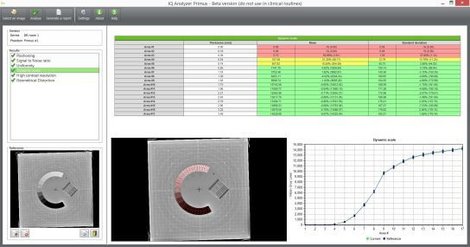
myQA® SRS is the first stereotactic detector with a large active detector area (12 x 14 cm2) to achieve film-class resolution of 0.4 mm. MatriXX ResolutionTM, the newest member of the well-known MatriXX detector family is a completely wireless IMRT/VMAT detector array with an impressive 6.5 mm resolution and more than 1500 ionization chambers. Both detector arrays feature the highest resolution available in the market for their specific field of use and do provide users with the capability to make pre-treatment patient QA in native plan geometry.
“Clinical proof, alongside close collaboration with clinical partners, has always been our driver to develop independent and innovative quality assurance solutions that give medical physicists around the world the accurate and efficient solutions they need to protect patients,”said Jean-Marc Bothy, President of IBA Dosimetry GmbH.“As a result, clinicians around the world have provided positive testimonials on our new detectors, proving that IBA is raising patient quality assurance in radiation therapy to a new level, enabling customers to increase the certainty of QA results, while significantly reducing the time required for patient quality assurance."
Ivo Petrov, Head of Medical Physics, Heart and Brain Center of Clinical Excellence – Pleven, Bulgaria said about the about myQA® SRS: “With myQA® SRS we evaluated routinely treated lung, liver, head and neck and paraaortic lymph nodes, gynaecological, head and neck recurrences SBRT cases. In almost all of the cases we’ve had strikingly good results with myQA® SRS providing similar results to multi-channel film dosimetry; with gamma pass rates of around 98-99% even for tighter gamma criteria such as 2%/2mm or 3%/1mm, for both FFF and FF beams. This makes us believe that the myQA® SRS system is a “game changer” in not only providing us with unmatched film-class resolution but also in reducing the number of false-negative and false-positive results in the patient pre-treatment QA process. With myQA® SRS we have the ability to perform QA easily for each case, something that is not possible with film dosimetry. This is further increasing our confidence in the quality of the advanced treatment techniques that we apply in our clinical center.”
The data follows IBA’s recent acquisition of Modus Medical Devices Inc, which with its complementary technology offering will allow IBA Dosimetry to market the most advance range of phantoms for radiation therapy quality assurance, strengthening its leading position in this segment.
About IBA
IBA (Ion Beam Applications S.A.) is the world leader in particle accelerator technology. The company is the leading supplier of equipment and services in the field of proton therapy, considered to be the most advanced form of radiation therapy available today. IBA is also a leading player in the fields of industrial sterilization, radiopharmaceuticals and dosimetry. The company, based in Louvain-la-Neuve, Belgium, employs approximately 1,600 people worldwide. IBA is a certified B Corporation (B Corp) meeting the highest standards of verified social and environmental performance.
IBA is listed on the pan-European stock exchange EURONEXT (IBA: Reuters IBAB.BR and Bloomberg IBAB.BB).
More information can be found at: www.iba-worldwide.com
For further information, please contact:
IBA
Christine Zollbrecht
Marketing and Inside Sales Director
Dosimetry-info@iba-group.com
Olivier Lechien
Corporate Communication Director
+32 10 475 890
communication@iba-group.com