Unlock the power of risk analysis and transform it into value!
With myQA PROactive, you will:
- Achieve higher safety levels
- Perform objective risk evaluation
- Strengthen compliance and follow best practice, efficiently
Contact us for your individual solution!
In the past, performing efficient risk analysis in a clinical setting was challenging.
With myQA® PROactive, you can now:
Ensure compliance and best practice
Align with key risk analysis guidelines, including AAPM TG 100 and European regulation 2013/59/EURATOM.
Start with fully editable, high-quality templates.
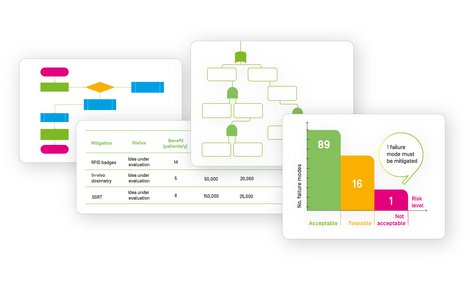
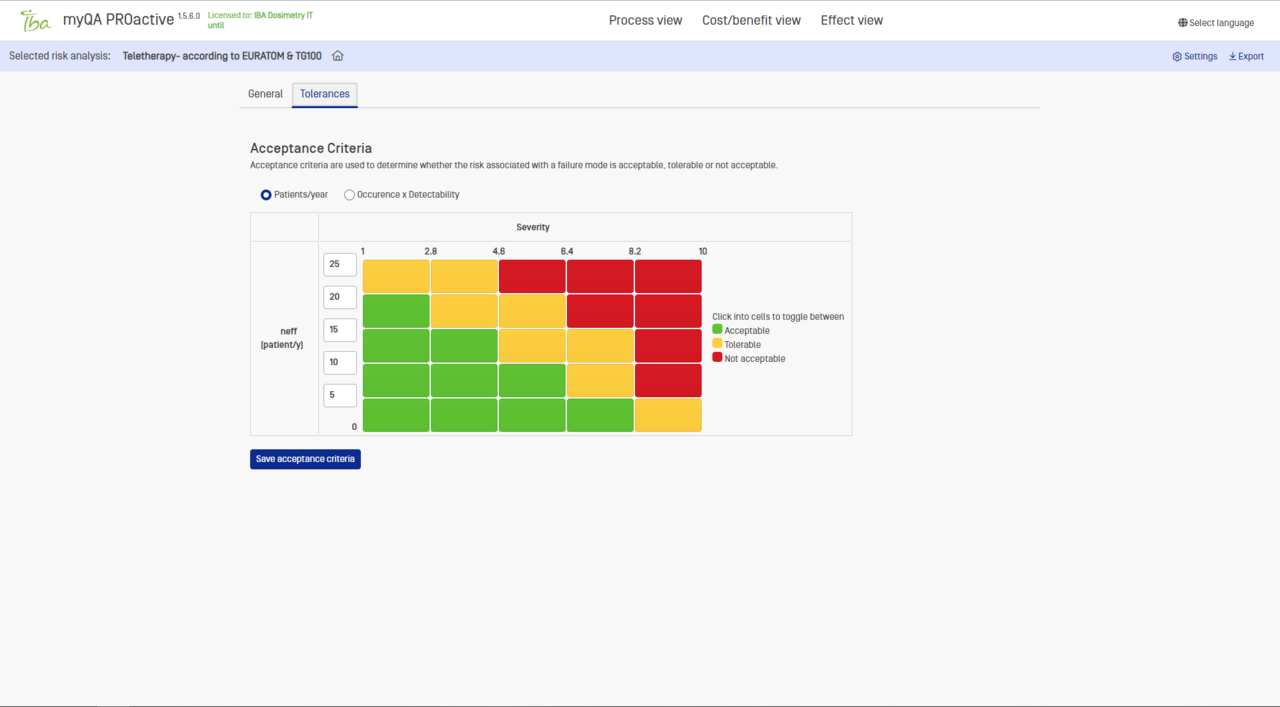
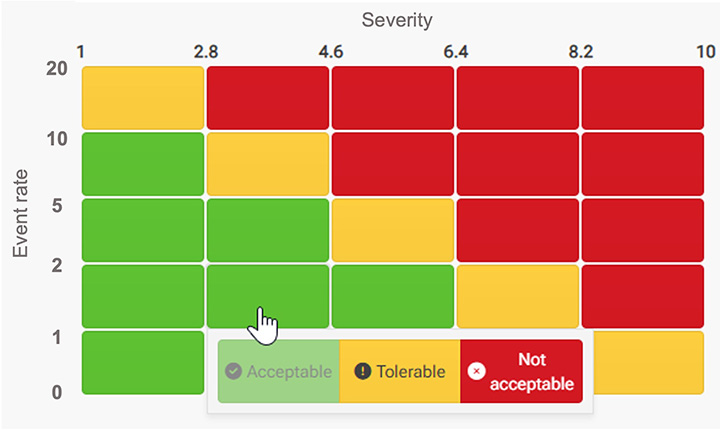
Evaluate risks using RPN and customizable risk matrices.
Generate customizable reports.
Enhance efficiency
Seamlessly import your existing FMEA Excel1 studies.
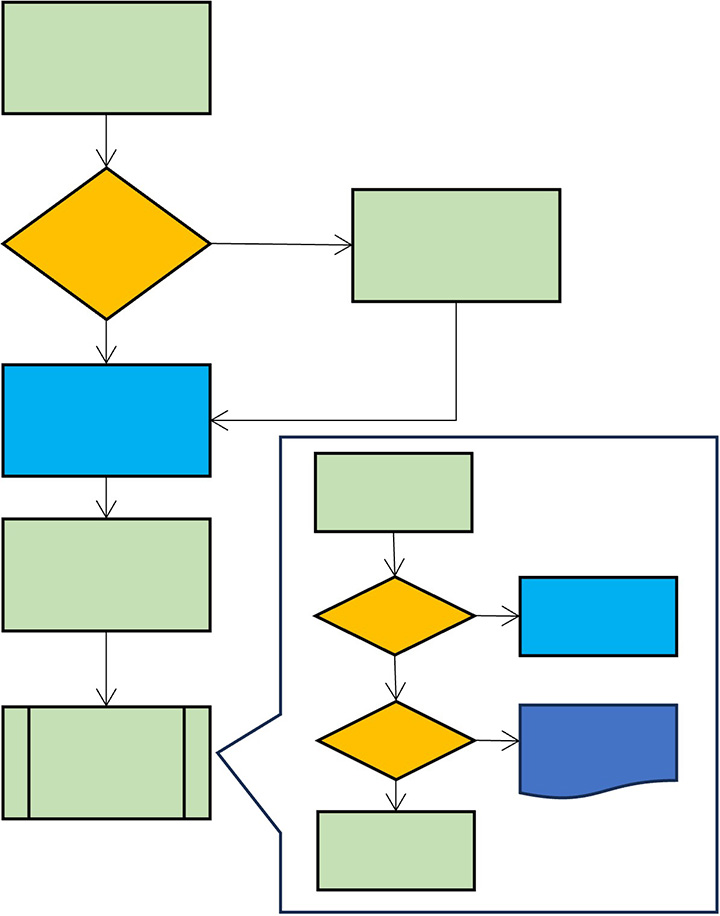
Facilitate team discussions with a user-friendly flowchart workflow description.
Maintain version control and electronic releases for ongoing risk analyses.
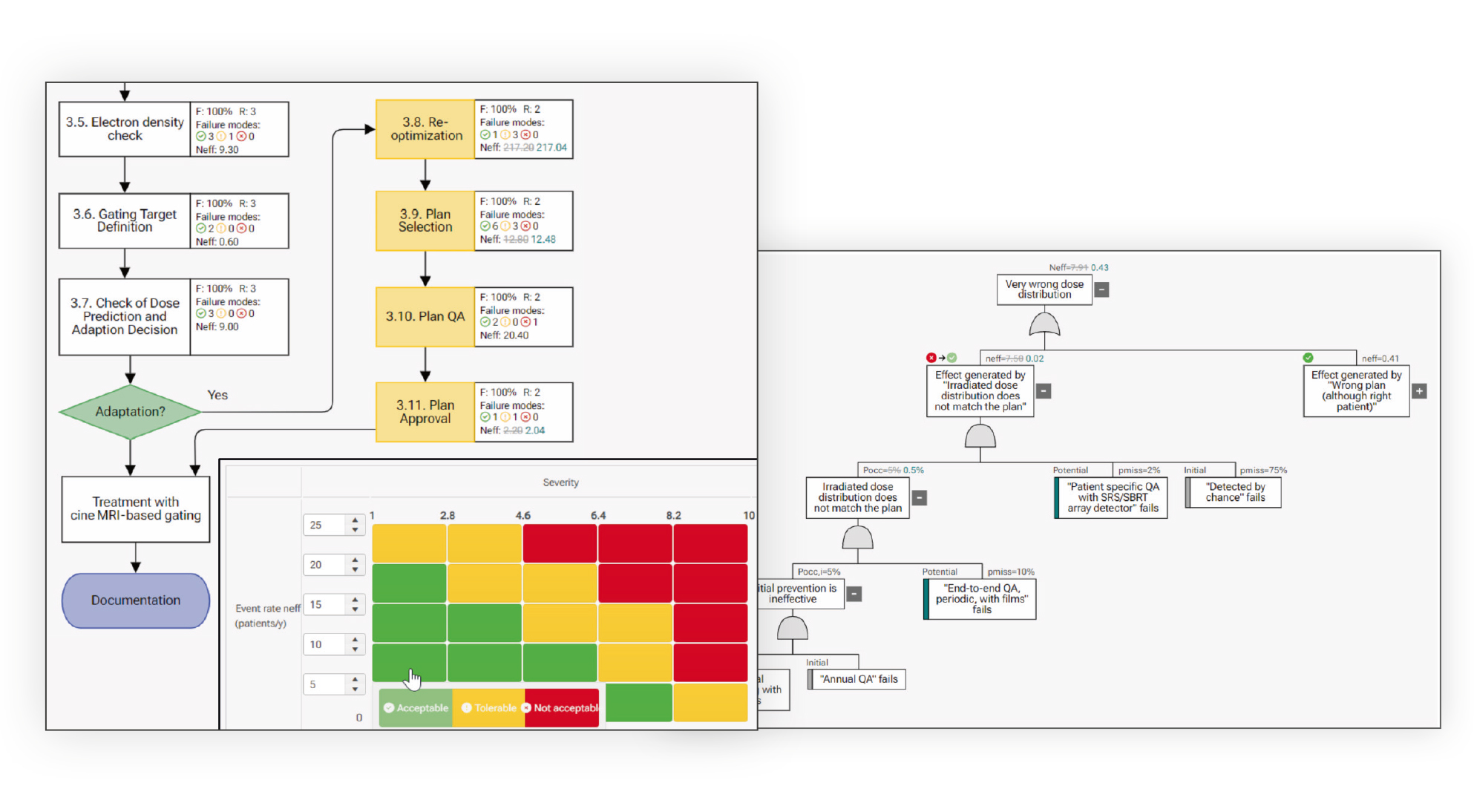
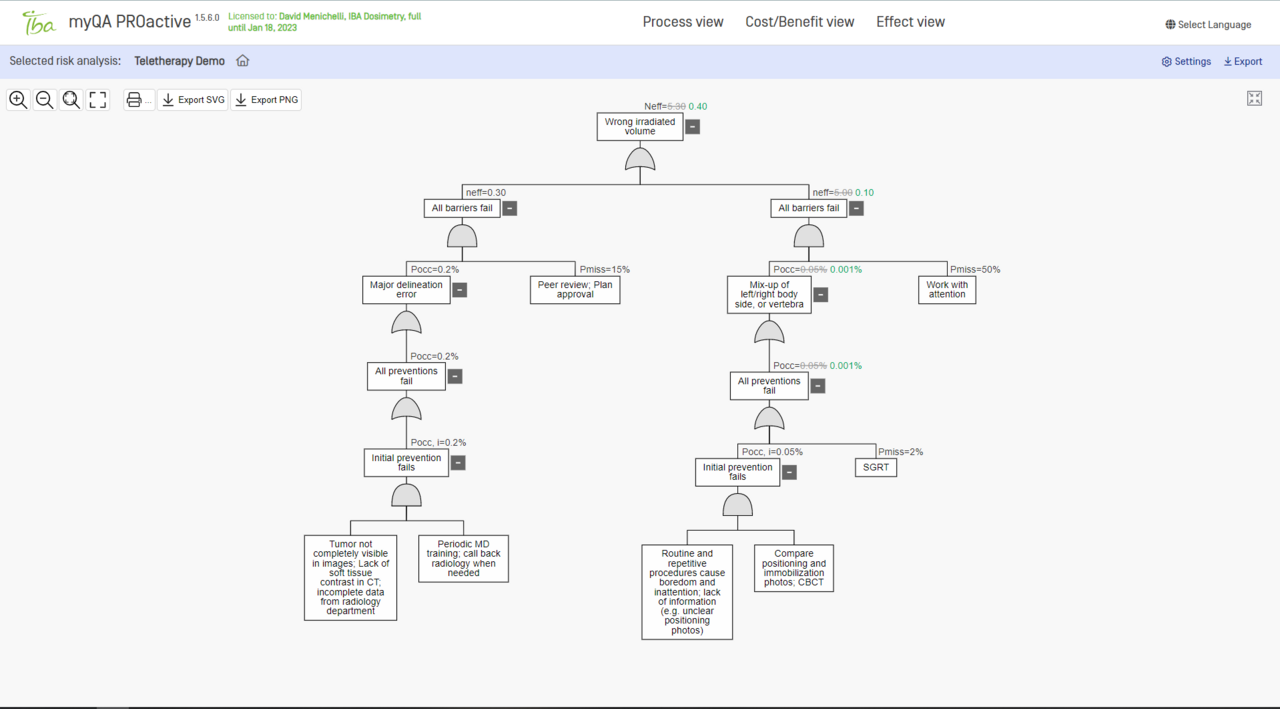
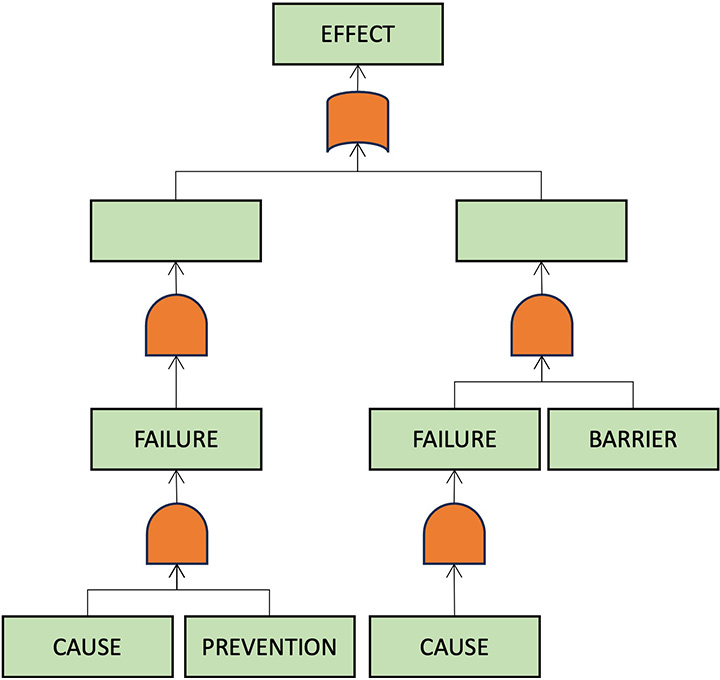
Focus on critical risks and explore failure causation chains with FTA risk analysis (automatically synchronized with FMEA℗).
Standardize risk management procedures across all relevant departments (RT, PT, nuclear medicine, etc.)
The only tool validating your risk analysis with real incidents
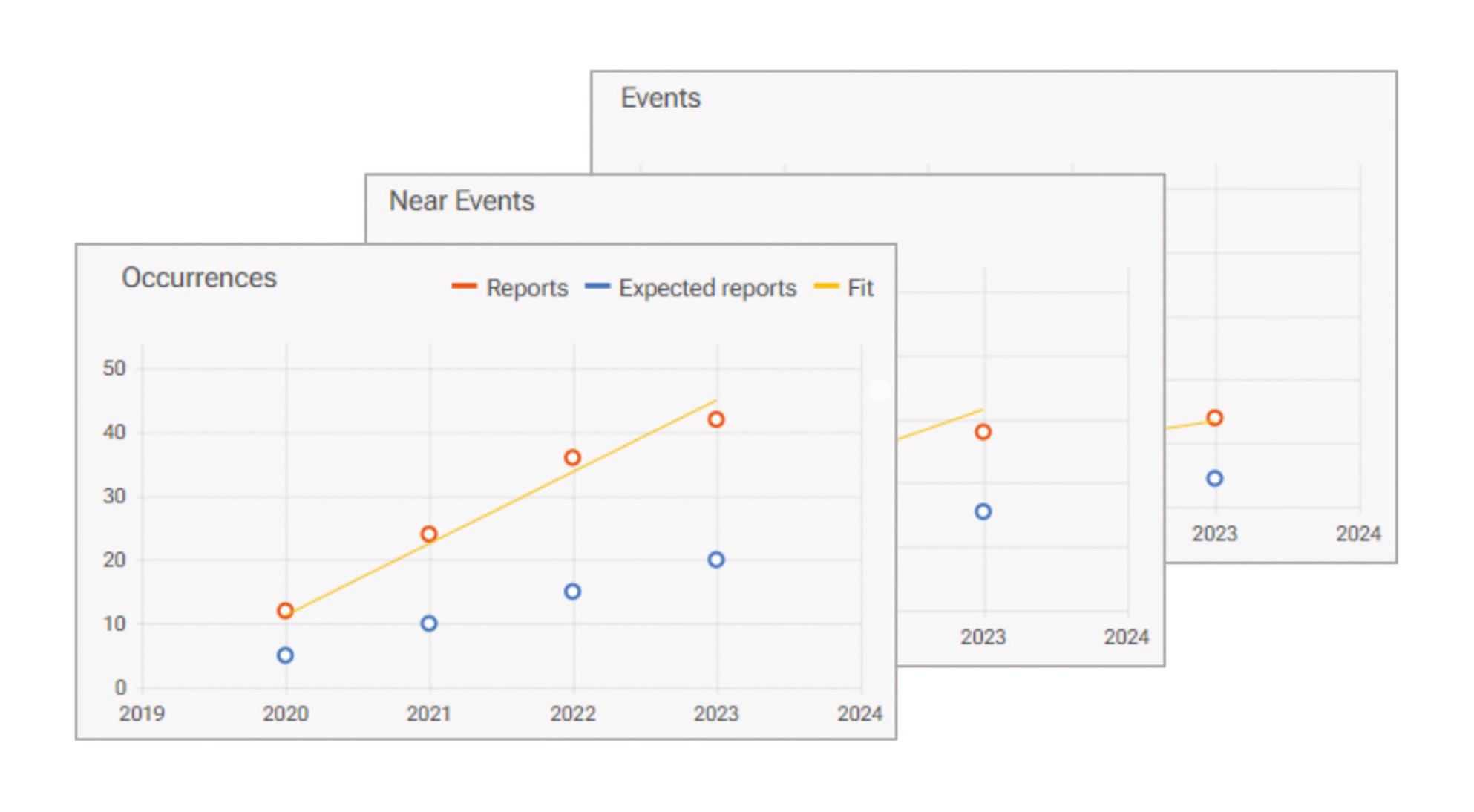
Implement a comprehensive risk management system you trust. It is finally possible to validate risk analysis predictions with reported incidents*. myQA® PROactive allows you to:
- Assess risks using "expected rate of adverse events."
- Easily report incidents from any device on your network.
- Analyze incidents in line with your policies, using customizable forms.
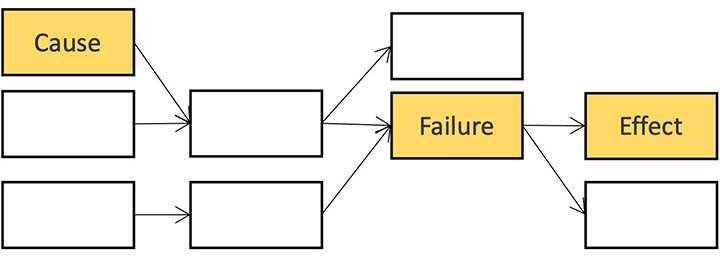
- Identify root causes and missing failure modes through “root cause analysis.”
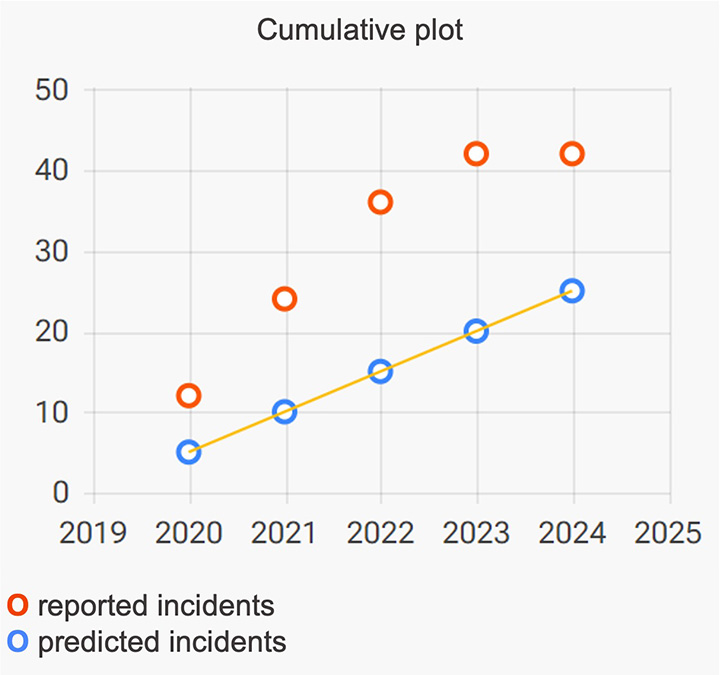
- Compare predicted and reported rates of adverse events and near misses.
- Adjust risk evaluation and achieve complete consistency between prospective and retrospective data.
- Evaluate safety measures based on the reduction in adverse event rates.
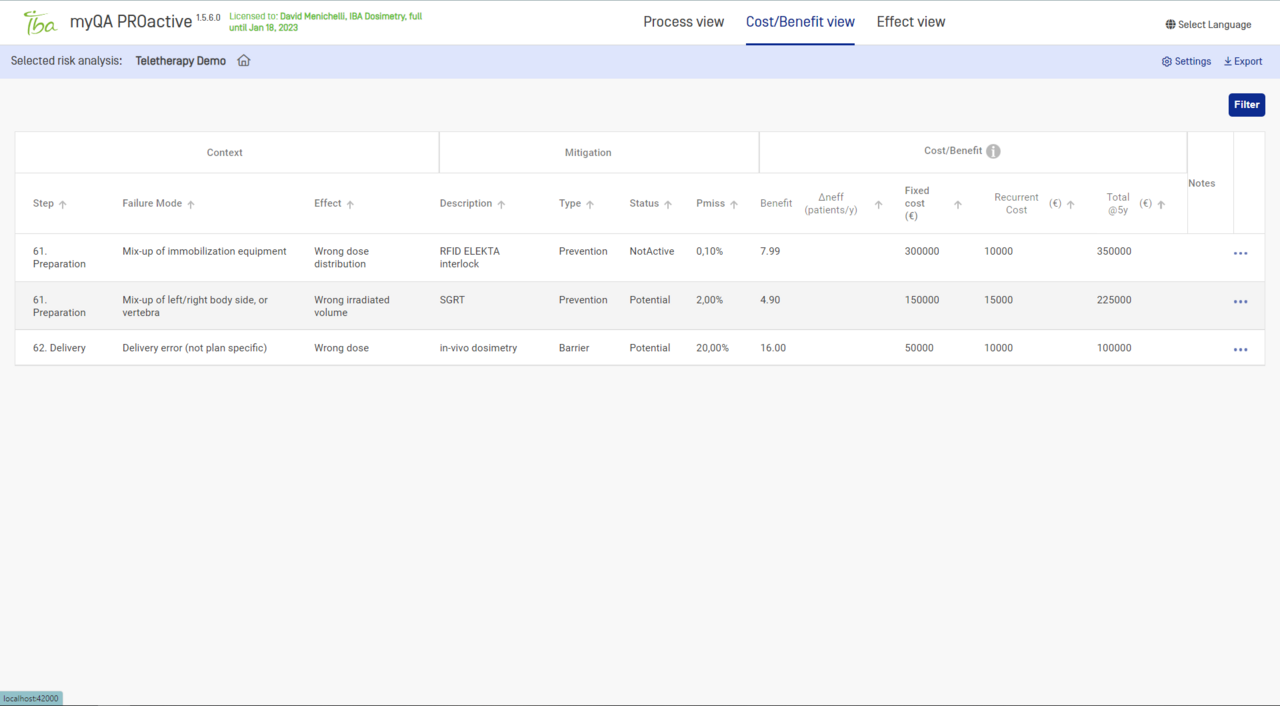
- Conduct a cost/benefit analysis of your improvement plan.
Compliance
Compliance with major risk analysis guidelines, including:
- AAPM TG 100;
- European regulations from the EU Council directive 2013/59/EURATOM.
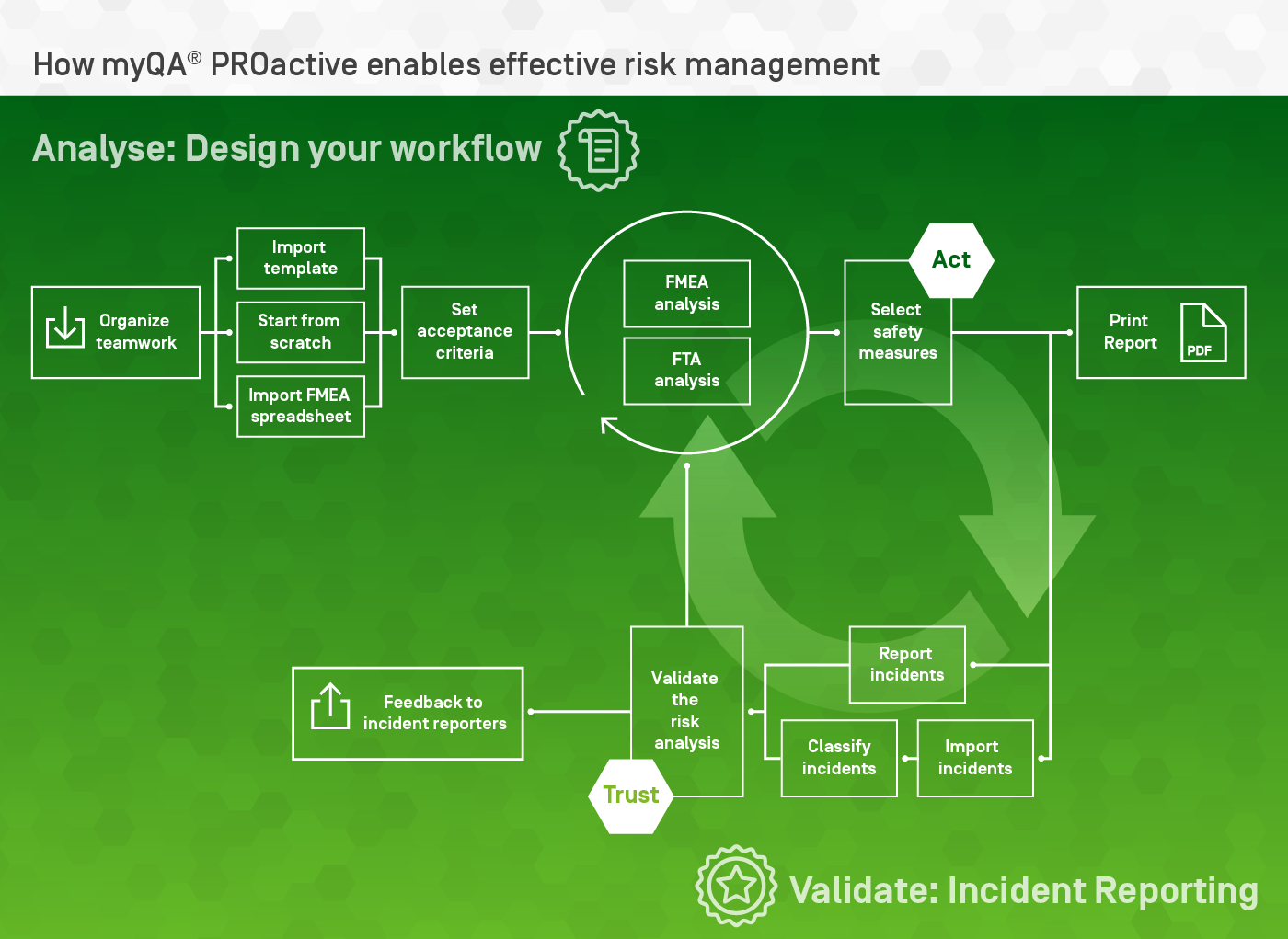
Organize teamwork
- Standardize risk management procedures across all relevant departments (RT, PT, nuclear medicine, etc.)
- Many users can work together using the browser-based interface
- View risk status with the library dashboard
- Maintain version control and electronic releases for ongoing risk analyses.
Acceptance criteria
FMEA
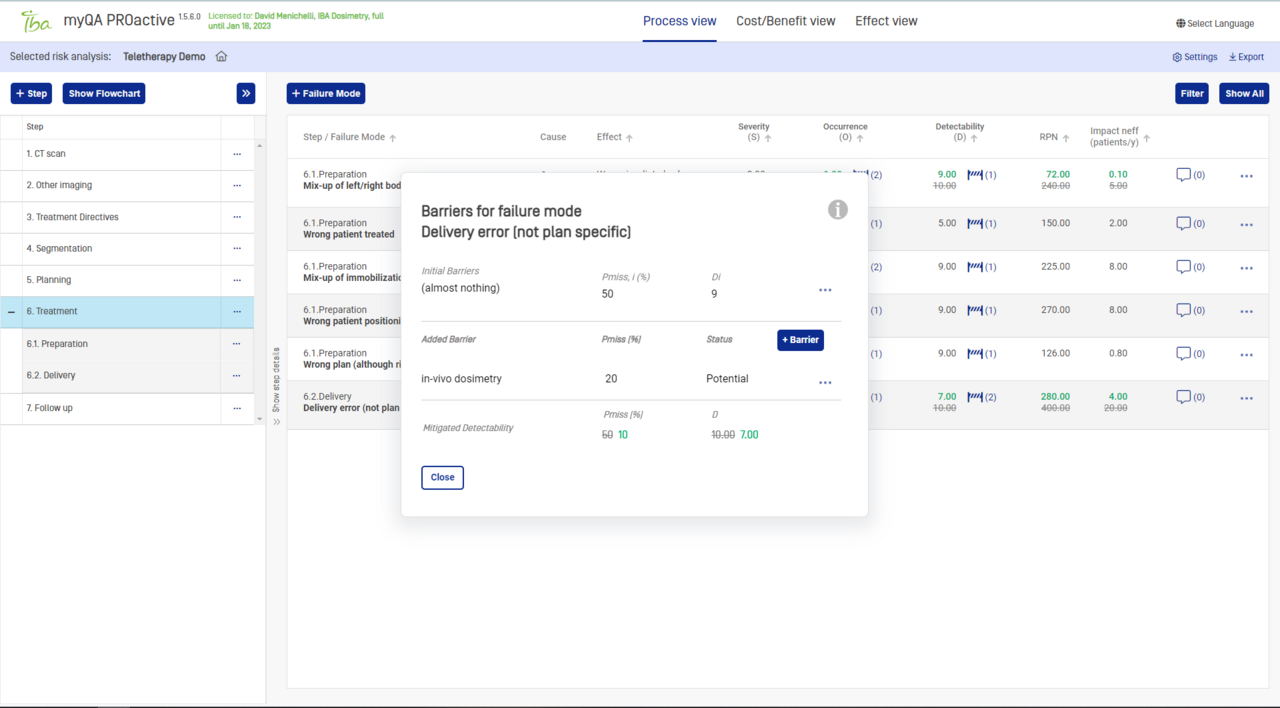
The Failure Mode and Effects Analysis (FMEA) method is an intuitive tool used by many risk managers. myQA PROactive starts you with a clean and powerful FMEA workspace where you can quickly learn how to use the innovative features of the SW.
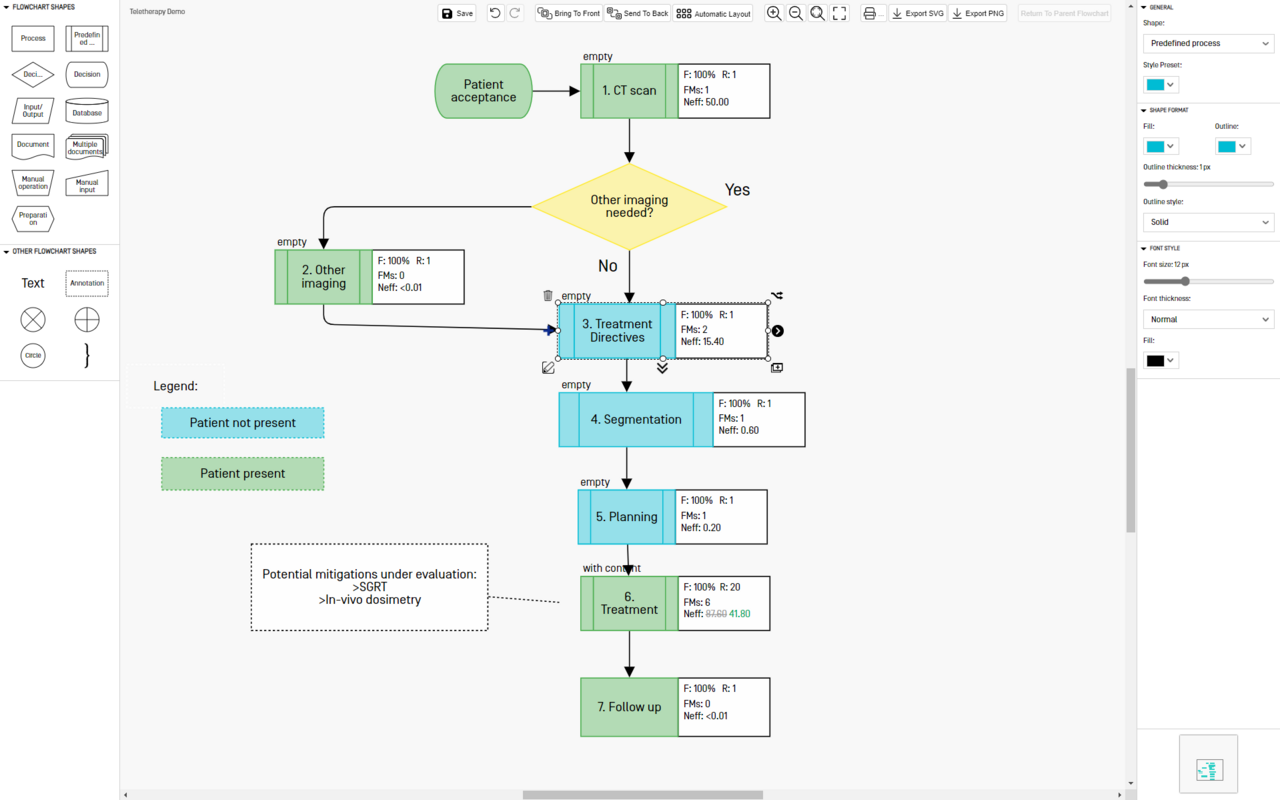
Generate flowcharts
Describe your workflow as a list or as a flowcharts. The two views are automatically synchronized and you can switch between them at any time.
FTA
FTA is a very efficient risk analysis tool focusing on worst-case scenarios.
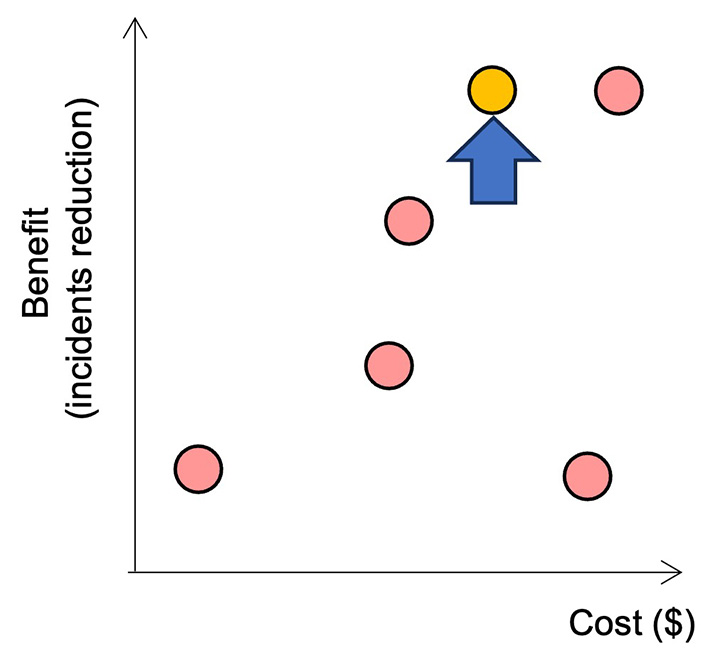
Cost-benefit analysis
Identify the most effective safety measures which can really boost your work-flow.
Risk analysis report
Generate customizable, high quality pdf reports.
Incident import
Are you reporting incidents with your IRL SW?
Import them into myQA® PROactive using its spreadsheet-based interface.
Incident reporting
Do you need an incident reporting SW?
- Easily report incidents from any device on your network.
- Analyze incidents with root cause analysis
- Generate documentation in line with your policies, using customizable forms.
Validate the risk analysis
Adjust failure modes evaluation in the risk analysis to achieve consistency between predicted and reported rates of incidents
Feedback for reporters
Show colleagues reporting incident how great they are!
myQA PROactive automatically generates a “gratification feedback” web page in the intranet
Reporters can see the status of the incidents they discovered. Sensitive information is automatically filtered out
FMEA vs FTA
FMEA and FTA can be alternative or complementary.
- FMEA is workflow centric and systematic but time consuming.
- FTA is patient centric and time efficient.
With myQA PROactive Synchronization℗ you can switch effortlessly between FTA and FMEA analyses at any time.
Proactive Risk Management in Radiation Oncology: from Burden to Value
Multi-professional healthcare teams use proactive risk management (RM) to analyse probable patient treatment failures and execute "safety measures" to prevent mishaps. IBA Dosimetry software for proactive risk management is myQA Proactive. This article shows how it solves FMEA spreadsheet flaws and facilitates AAPM TG100 [1] implementation so every clinic may manage risks with a positive effort balance.


"myQA PROactive is an innovative tool for prospective risk analysis tailored to the needs of radiation oncology. It offers a formalized approach to risk assessment following best practice methodology. The software includes flowcharts and FMEA, and the integrated fault tree analysis identifies measures to block multiple error pathways, further increasing patient safety."
Prof. Dr. rer. nat. Christoph Bert, Head of Medical Physics, Erlangen University Hospital
Clinical References
Technical specs
Specifications - General
Deployment | On-premise hosting with Kestrel web server |
Access | Web browser based; multiple users; multiple “departments*” |
Data I/O | xls, xml, pdf, json |
Specifications - Risk analysis
| Risk analysis techniques | FMEA & FTA (automatically synchronized) |
| Process description | List & Flow chart (automatically synchronized) |
| Risk evaluation | Risk matrix, RPN, expected rate of adverse events |
| Decision tools | Cost/benefit analysis; mitigation scenarios |
| UI language | Customizable |
| Version control | Multiple versions for any risk analysis; electronic release |
Specifications - Retrospective module*
| Incident reporting | Reporting terminals; application UI; xls |
| Warnings | Via email |
| Incident documentation | Root cause analysis; customizable forms |
| Risk analysis validation | Comparison of expected and reported adverse events and near misses on failure mode basis |
1. Windows 10, Windows Server 2016, and Edge are trademarks of the Microsoft group of companies.
2. Firefox is a trademark of the Mozilla Foundation in the U.S. and other countries.
* Release pending
System requirements
Installation machine | PC (single user) |
Operating system | Windows 10 1 or newer |
Minimum CPU power | 2x2.0 GHz |
Minimum available RAM | 8GB |
Minimum free disk space | 4GB |
Minimum screen resolution | 1650 x 1050 |
Supported browsers | Edge1, Chrome™, and Firefox2 |
| Supported databases | SQLite, Microsoft SQL Server* (2016 or newer) |
* Release pending